TORONTO -- Health Canada says companies involved in the compounding and admixing of medications can continue to do so, but under new oversight conditions.
The conditions are a temporary fix to a jurisdictional grey area believed to have contributed to more than 1,200 patients receiving diluted chemotherapy drugs.
Dr. Supriya Sharma, senior medical adviser with Health Canada, says the conditions will allow companies to continue providing services while Ottawa and the provinces and territories work out a long-term oversight plan.
Compounding and admixing can continue if it is done within a hospital meeting provincial requirements, outside a hospital under the supervision of a provincially licensed pharmacist, or in a manner that meets the licensing and manufacturing requirements of the Food and Drugs Act.
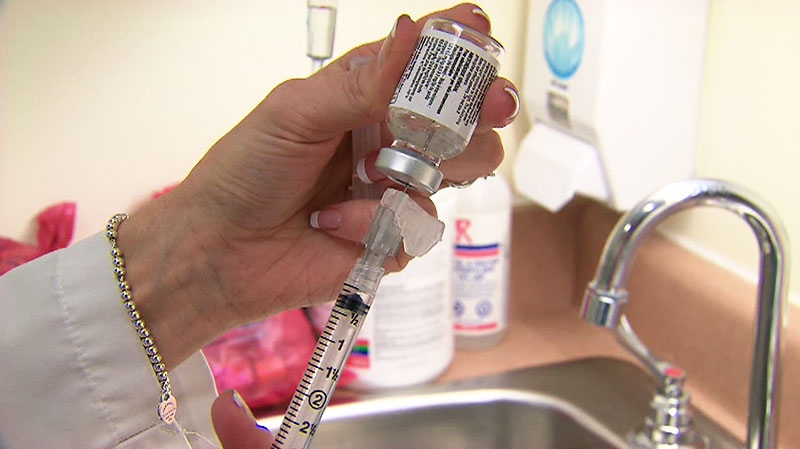
Marchese Hospital Solutions provided the watered down drugs to five hospitals in Ontario and New Brunswick, but has said the problem was with how the drugs were administered at hospitals.
However, the new conditions might not have prevented the incident, as Sharma says Marchese is believed to have been working under appropriate oversight.
"From the information that we have obtained from Marchese Hospital Solutions, we do believe that they had appropriate oversight in place," Sharma said Friday.
Marchese Hospital Solutions, which had a pharmacist on staff, fell into a jurisdictional grey area, with Health Canada and the Ontario College of Pharmacists unable to agree on who was responsible for the facility.
Health Canada regulates and inspects drug manufacturers, while the college is responsible for pharmacists in Ontario, including those who may have been working independently for the company. Hospitals are responsible for the purchase and security of their drugs.
Too much saline was added to the bags containing cyclophosphamide and gemcitabine, in effect watering down the prescribed drug concentrations by up to 20 per cent. Some patients were taking the drugs for as long as a year.
Sharma said Canadians can be reassured that organizations following the new guidelines "will have the active oversight in place to help ensure the safety and effectiveness of health products prepared in this way."
The Ontario government said Friday it is posting a new regulation under the Public Hospitals Act to ensure that hospitals purchase drugs only from accredited, licensed or otherwise approved suppliers.
The province is also working with the Ontario College of Pharmacists on a regulation to give the college the power to inspect premises where pharmacists and pharmacy technicians practice, including where drugs are prepared.
The government is also contacting businesses in Ontario that may be selling compounded drugs to obtain more information about their activities.
All Ontario hospitals have been asked to confirm that quality assurance processes are in place for all drugs purchased externally or prepared in the hospital.
"Ontario's actions, combined with Health Canada's commitment, will ensure that patients in Ontario hospitals can have full confidence in the safety of their drug treatments," Deb Matthews, Minister of Health and Long-Term Care, said in a statement.
"However, this is national problem, and the federal government has an important responsibility in providing a long-term solution," Matthews noted.
Ontario NDP health critic France Gelinas said the federal government has now made it clear who is responsible for drug compounding and admixing companies.
"The federal government made it really clear their oversight is going to stop at manufacturing -- everything else is the responsibility of the province," Gelinas said.