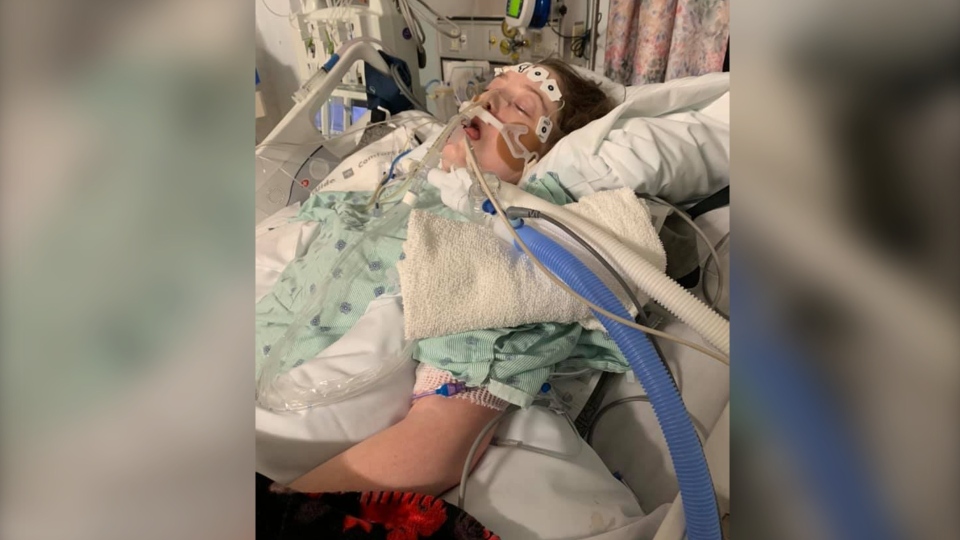
HALIFAX -- The death of a 23-year-old Nova Scotia woman with cystic fibrosis is galvanizing a movement led by CF patients across the country to fight for access to the drug that could have saved her life.
Chantelle Lindsay never wanted to bring attention to her diagnosis, but her death has become a rallying cry for others fighting the fatal genetic disease.
Chantelle died Feb. 19 after being in the QEII hospital for almost six weeks. Her lung function started decreasing last fall, and she was admitted to hospital in late December.
Chantelle’s family says her medical team had applied to a U.S. drug company, Vertex Pharmaceuticals, to gain access to a new breakthrough drug, Trikafta, through compassionate use. After hearing nothing for a month, the family reached out to Health Canada for help.
Chantelle’s condition worsened to the point that she was put on a ventilator, and Vertex denied her claim. Chantelle died in hospital less than a week later.
Chantelle's legacy
Cystic fibrosis is the most common fatal genetic disease for Canadian children and young adults. The disease affects the patients’ digestive system and lungs, until the lungs eventually stop working.
Chantelle was diagnosed with cystic fibrosis as a child, but her father, Mark Lindsay, says she never let it stop her from living life to the fullest.
She was a welcoming person, he says, who was always taking others “under her wing.”
Hundreds of people attended her memorial Wednesday at First United Church in Truro, N.S. Lindsay says there were so many people in attendance, he spent three hours in the receiving line.
People from all over the country have reached out to Chantelle’s family, touched by her story.
“She’d be a little embarrassed at first, because she never wanted a lot of attention,” Lindsay says. “But I think she’d be pretty proud.”
“She did say something in the hospital about being a bit of a ‘celebrity’,” he adds. “I think she’d be pretty proud that we’re still fighting for her, and what this has actually brought out….It’s been pretty touching.”
Inspiring CF 'warriors'
Tim Vallillee never had the chance to meet Chantelle. The 52-year-old from Yarmouth, N.S., also has cystic fibrosis. Eight years ago, he fought to gain access to another drug manufactured by Vertex, called Kalydeco. He finally got it in 2014. The drug is now covered by Nova Scotia’s provincial drug plan. Vallillee says the medication has changed his life, doubling his lung function, which was around 30 per cent at the time.
As a fellow CF patient, Vallillee connected with Chantelle online, after she commented on one of his Facebook posts. While he wanted to visit her in hospital, he couldn’t because of something all cystic fibrosis patients know: they have to stay at least six feet away from each other, to prevent transmittingpotentially deadly infections. So Vallillee made a balloon bouquet for Chantelle instead – it was taken to the hospital shortly before she died.
“The fact that this [Trikafta] is the fourth drug to come out, and it’s still a battle to get this into the hands of people that need it, like Chantelle, it is beyond frustrating,” he says.
Vallillee is now organizing a Halifax rally at Province House in Chantelle’s name to bring attention to the issue. The event on March 25 will coincide with a series of rallies across the country.
“I hope in my heart of hearts,” he says, “that the politicians, and the drug companies, and whoever is in charge of these decisions, hears the stories of people who are living this, and something in their heart goes, ‘I need to put my name on this, why are we wasting time?’”
'Gambling with the lives of Canadians'
The catch with Trikafta is that Vertex hasn’t even applied to have the drug approved for use in Canada yet. The drug has been approved in the U.S. and the U.K, among other countries. The drug has been hailed as a “breakthrough” which can treat the underlying cause of CF for up to 90 per cent of patients.
When Chantelle’s medical team applied to the drug company for compassionate use, it also applied to the federal health department’s “Special Use Program.” Lindsay says Health Canada approved that application, but under the rules of the program, the drug manufacturer has the final word on whether the medicine can be administered.
While Vertex won’t comment on Chantelle’s case due to “privacy considerations,” Lindsay says Vertex denied the application because by that time, Chantelle was already on a ventilator and was no longer considered a candidate for Trikafta.
The company hasn’t explained why it hasn’t applied to have the drug approved in Canada yet, but did say in a statement, “Vertex has strong concerns that new Canadian medicine pricing rules just introduced, as they are currently written, have the potential to limit access to treatments for Canadians living with a rare disease.”
Vertex is referring to Canada’snew regulations for medicines under patent protection – rules introduced to changehow the Patented Medicine Prices Review Board (PMPRB) negotiates drug prices. Health Canada says the changes “will make prescription drugs both more affordable and more accessible for all Canadians, saving an estimated $13 billion in the next decade.”
Dr. John Wallenburg, chief scientific officer for Cystic Fibrosis Canada, says the new rules also have a serious side effect for Canada’s 4,300 CF patients, creating a “chill effect” among pharmaceutical companies balking at submitting their drugs to Canada.
“The federal government and Health Canada have been telling us for a couple of years, “No, it’s OK, we can reduce prices, and the process that we’ll put in place won’t affect access to medicines,’” he says.
“They’re calling the bluff of the industry,” Wallenburg adds. “But when you’re doing that, you’re gambling with the lives of Canadians. You can’t do that.”
On Feb. 14, Cystic Fibrosis Canada made a submission to the PMPRB, asking it to hold off on implementing some of the drug pricing policy changes. It will meet with the board Monday to discuss its concerns.
Fighting for access to other breakthrough CF drugs
When Beth Vanstone of Beeton, Ont., heard about Chantelle’s passing, she cried.
“Because that could be my kid, and we don’t live in a country that lets that happen, or we shouldn’t,” she says.
Vanstone’s daughter Madi also has CF. But the 18-year-old is on the same drug as Tim Vallillee, Kalydeco. Like Vallillee, Vanstone fought the Ontario government’s refusal to cover the drug and won.
Vanstone is part of the “CF Treatment Society,” a grassroots group advocating for better access to medications. She was among CF activists, parents, and patients who attended question period at Queen’s Park in Toronto last Wednesday to pressure Ontario Minister of Health Christine Elliott to publicly fund cystic fibrosis drugs.
Along with Trikafta, Vertex is the maker of Orkambi and Symdeko –part of the same series of breakthrough “gene-modulator” CF medications. All cost around $300,000 a year. Orkambi and Symdeko are approved for use in Canada, but are not covered by public health care.
Chantelle was on Orkambi for more than a year and a half. But she stopped taking it, after she became too old to be covered by her father’s private insurance when she turned 22.
“It’s really infuriating to me,” Vanstone says. She firmly believes Chantelle’s condition would not have declined so quickly, if Orkambi was covered by provincial health care.
“We’re going to our provincial government, because what we’re pushing for…is getting a portfolio deal,” she explains. “That means it would cover all the drugs, and all the new drugs coming in. One negotiation, one price, and stop all the time that’s being wasted.”
She says provinces can negotiate their own deal with drug companies like Vertex through the pan-Canadian Pharmaceutical Alliance (pCPA). That way, she says, provinces don’t have to wait for action from the federal government, as long as the drug is approved for use by Health Canada.
Vanstone is critical of Ottawa’s new drug pricing proposals.
“The new [plan] the federal government is coming out with, where they’re going to save a few pennies on antibiotics or aspirins, they’re saving that money and they’re leaving all the rare disease patients out to dry,” she says.
'Get loud'
Since Chantelle’s passing, her story has spread on social media in a wash of purple ribbons, stickers, and wreaths, motivating many people to add their voices to an awareness campaign led by those affected by cystic fibrosis, called “CF Get Loud.”
But even if the PMPRB puts a hold on some of the drug policy changes, and Vertex applies to have Trikafta approved for use in Canada, Wallenburg says it would likely take several years to get the cost of the drug covered for Canadians.
In the meantime, those in the community where Chantelle lived are holding various fundraisers to help the family in its time of grief.
Mark Lindsay says he will keep fighting in in his daughter’s name.
“I want to see [us] get this drug in Canada, and have somebody cover the cost of it,” he says.
I don’t know the answer, who is going to pay for it, but they gotta get it figured out.”