FREDERICTON -- An Ontario-based company that pays people to donate blood plasma -- a practice banned in two provinces -- is set to make New Brunswick its next centre of operation, fuelling an ongoing ethical debate.
Canadian Plasma Resources is looking to western and Atlantic provinces, including New Brunswick and Nova Scotia, after legislation in Ontario shuttered their two clinics in Toronto in 2014. Paid plasma donations are also not allowed in Quebec.
Company CEO Barzin Bahardoust said at least 80 per cent of plasma protein products in Canada are imported from jurisdictions that compensate plasma donors.
"So to me the compensation is not really an ethical question as long as the donor is informed and consents and the patients that receive these products are aware of where they are coming from and how they are manufactured," he said.
Donors can give plasma once a week, and Canadian Plasma Resources gives them either a charitable tax receipt or a reloadable gift card in the amount of $25.
But the issue of paying people for their donation raises an ethical question and is stirring some strong opposition.
"The idea of commodifying a part of a person in this way is quite repugnant," said David Coon, leader of the New Brunswick Green party.
Coon supports maintaining a voluntary system of blood and plasma donation through Canadian Blood Services.
"The same holds for organs, tissue, for sperm, for eggs and this would be a dramatic departure from that if New Brunswick permits this to go ahead. Both Ontario and Quebec have outlawed it and for good reasons in my opinion," he said.
New Brunswick Health Minister Victor Boudreau said he welcomes a Canadian Plasma Resources clinic as long as it meets all the regulations set by Health Canada.
"There are other provinces, Manitoba allows paid blood plasma clinics as does Saskatchewan and there are other provinces in discussion with Canadian Plasma Resources as well," Boudreau said.
Coon wants the issue debated in the legislature, but Boudreau said there's no need for that.
"There is no law in New Brunswick that prevents it...We currently purchase plasma that was paid for in the U.S. I find it a little hypocritical if we're saying we can't pay Canadians to collect plasma, but we can pay Americans to collect plasma," he said.
But Francoise Baylis, a professor and Canada Research Chair in bioethics and philosophy at Dalhousie University in Halifax said there are many, including the World Health Organization and the Krever Commission's report on Canada's blood system that would disagree with Boudreau's stance.
"Is this same government prepared to start paying people to donate their organs because we have lots of Canadians that could benefit from organs, same as we have lots of Canadians who would benefit from blood and plasma? Where do you want to draw the line?" Baylis asked.
"I am a defender of the altruistic system and I would not support the payment for anything whether it be sperm, eggs, blood, plasma, etc."
The World Health Organization has argued for 100 per cent voluntary, non-remunerated donations by 2020.
"Why would Canada be pulling in the opposite direction?" Baylis said.
In 2014, Deb Matthews, who was Ontario's health minister at the time, said legislation to prohibit paid donations was intended to preserve the integrity of the province's voluntary blood and plasma donation system.
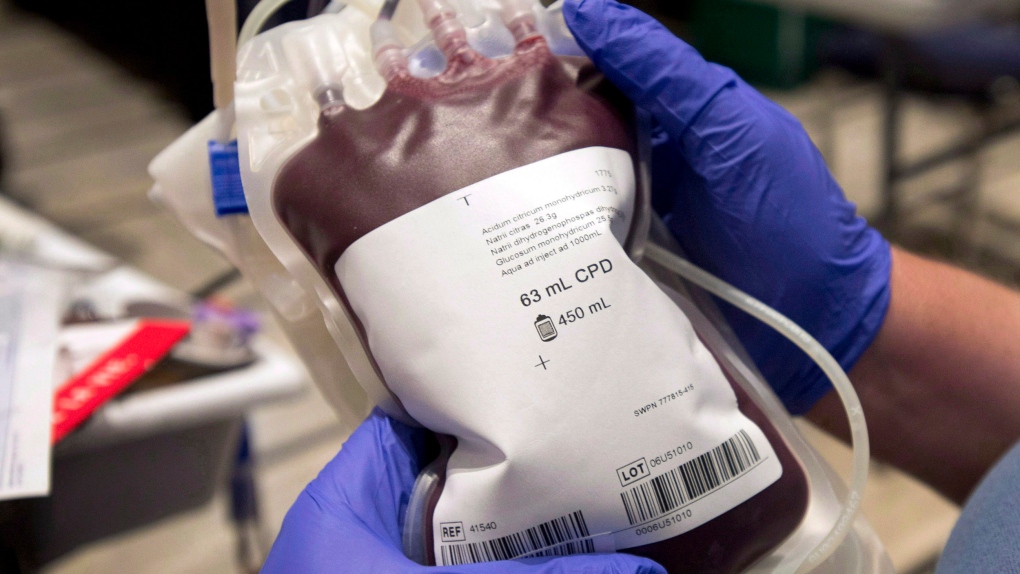
Blood plasma is the yellowish fluid that remains after red and white blood cells and platelets are removed.
Fresh plasma is used for transfusions while processed plasma is turned into a variety of pharmaceutical products. Plasma collected in Canada is sent to the United States for processing and then purchased back from American companies as end products.
Canadian Plasma Resources now has a clinic in operation in Saskatoon. Bahardoust said a New Brunswick clinic, likely in Moncton, would have 30 donation beds and create 40 full-time jobs.
Bahardoust said he has not asked for any financial assistance from New Brunswick, but has been dealing with Opportunities New Brunswick which often provides payroll rebates for new jobs created.
"We are hoping that we will qualify, but nothing has been finalized at the moment," he said.
Bahardoust said once a site is selected it should take about six months to get it ready and get approval from Health Canada.