TORONTO -- Women who may have taken birth control pills from faulty packs should use a backup form of contraception for the time being, the Society of Obstetricians and Gynecologists of Canada suggested Wednesday.
And women may need to talk with their doctors about taking a pregnancy test, said Dr. Jennifer Blake, the organization's CEO.
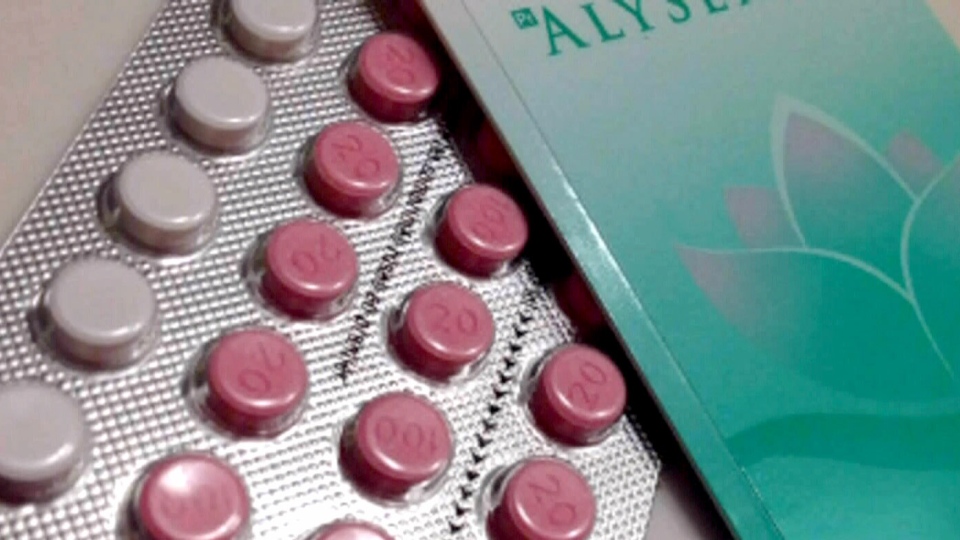
The advice is prompted by a major birth control recall involving the drug Alysena 28. One lot of the product, bearing the number LF01899A, contained too little active drug and too much placebo, leaving women who took it vulnerable to becoming pregnant.
The lot, which contained about 50,000 faulty packets, was distributed across Canada. The company that sells the product in Canada, Apotex, believes it was not distributed to Alberta, Saskatchewan, Manitoba, Northwest Territories and Nunavut, but it cannot say with certainty that that is so, said Elie Betito, the company's director of public and government affairs.
Women using birth control pills take the drugs for 21 days each menstrual cycle. Because of the risk that they might not remember to resume taking their pills at the right time, many oral contraceptives are packaged with a pill for each of the 28 days in a cycle -- 21 are drug and seven are placebos.
Blister packs in this faulty lot contained 14 pink pills containing active drug and 14 white placebos. And just that seven-day difference could be enough to result in a pregnancy, Blake said in an interview.
"That would mean two weeks without medication and that certainly gives enough time for the ovary to release an egg," she said. "And therefore if you have been taking that particular pill, Alysena, then it is important that you let your doctor know and indeed it may be important to follow up with a pregnancy test."
The problem is, some women may not know they were taking this contraceptive. That's because the drug is a generic version of the most popular birth control pill used in Canada, a drug called Alesse. (Alesse is not involved in this recall, Blake stressed.)
Some prescription drug plans require pharmacists to replace higher-priced brand-name drugs with generic versions if a generic is available. That may make it difficult to get a sense of who was on this drug, Blake says. It may also mean that women might think they are taking Alesse, because that is what their doctor prescribed, but they are actually taking Alysena.
"The problem is that it's a generic copy of the leading birth control bill. So that's a problem because we don't know how many women have had their prescription substituted for the generic. And women may not know they've had their prescription substituted, because the generic chose a very similar name and look to the leading birth control pill. And that does create confusion in everyone's mind."
Blake said women who take this contraceptive should be using other methods of birth control as well until they've gone a full month on a proper packet of the drug. "I would regard it as being like a new start" (on oral contraceptives).
"If you have started a packet of Alysena this month, if that's what you're taking now, then you should be using an additional method and calling your doctor," she said.
"If you took the faulty packet last month and you got your period, then that's very reassuring. If you took the faulty packet last month and you did not get your period, then you certainly need to be getting a (pregnancy) test."
Blake said Plan B, the so-called morning after pill, would also be an option for women who have had what amounts to unprotected sex because they were on a faulty pack of Alysena. But the drug only works within three to five days -- and five is pushing it -- after unprotected sex.
Alysena is manufactured in Spain by Leon Farma for Apotex. Betito said the company is working with the manufacturer to try to figure out what went wrong.
Health Canada also wants answers to that question, said Dr. Supriya Sharma, senior medical adviser for Health Canada's health products and food branch.
"There does look like there was a problem in their good manufacturing processes. Otherwise this wouldn't have occurred," Sharma said.
Apotex notified Health Canada of its intention to recall the product on April 3. As required, it submitted a full risk assessment to the department by late in the day April 4. Health Canada finished assessing the file Monday morning, and posted a notice of the recall on its website.
The company felt the risk assessment for the recall was a Type 2 under Health Canada's rules -- something that might cause temporary adverse health effects if used. But Health Canada told the company it felt this merited a Type 1 designation. A Type 1 risk is reserved for drugs that could cause serious injury or even death if used.
Sharma said that while for many Alysena users the risk was lower, some women using the drug might have been warned not to become pregnant because it might endanger their health or because they were on medication that would endanger the health of any fetus they conceived.
Recalls with a Type 1 designation involve notifying pharmacists and getting them to pass the message along to patients or to the prescribing doctor.