A birth control pill has been pulled from pharmacy shelves for the second time in five months and university students say the recall couldn’t have come at a worse time.
“At least if it was a normal time of the semester people would be paying more attention with the news but now people are just looking to have a good time and not really listening to what’s going on,” says Dalhousie University student Schona Grimes.
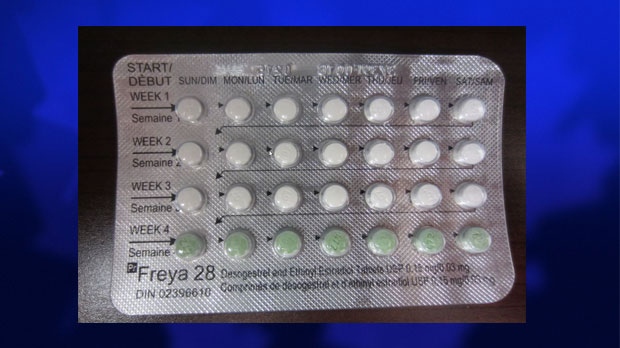
Health Canada says a placebo pill was found in place of an active one in a package of the oral contraceptive Freya-28.
Packages of Freya-28 have three rows of white, active pills, along with one row of green, placebo pills. Women typically take 21 active pills, followed by seven placebo pills during their menstrual period.
Two Freya-28 lots were affected: 3739F001B and 3739F002B. The company’s other product, Freya-21, which contains only 21 active pills, are not part of the recall.
Almost 76,300 packages from the affected Freya-28 lots have been distributed across Canada since May 10 -- meaning that potentially thousands of women are affected.
The drug is packaged for Mylan by a Mumbai, India-based pharmaceutical company called Famy Care Ltd.
Health Canada, which is monitoring the voluntary recall, says missing one or more of the active pills could result in reduced effectiveness of the contraception and risks the possibility of an unplanned pregnancy.
However, Dr. David Juurlink says it is highly unlikely women who took the faulty pill will become pregnant.
“In my estimation, if this is a single tablet issue, even with a large number of packs, this is…not catastrophic by any means,” he says.
But university students in the Halifax area say the incident is concerning.
“I just think a lot of girls put their trust in birth control, especially if it’s recommended to them by a doctor,” says Dalhousie University student Liz Moumouris. “That could seriously mess up someone’s life.”
“If birth control isn’t working for them then, wow,” says student Myles Braithwaite. “Something I should definitely have consideration towards to know whether or not I should be using protection or not using protection.”
Unopened packages should be returned to the pharmacies where they were purchased, Health Canada says.
Health Canada also recommends that users of the pills use a non-hormonal method of birth control, such as condoms, spermicidal foam or gel, until another oral contraceptive can be obtained.
Consumers with any further concerns should contact their healthcare practitioners. Users of the pills can also contact Health Canada's toll-free line at 1-800-267-9675 with questions or complaints.
Consumers are also being asked to report to Health Canada any problems they might have potentially related to recalled pills, by visiting MedEffect Canada's webpage on Adverse Reaction Reporting.
The recall comes just four months after another similar birth-control pill recall, involving a brand called Alysena 28. One lot of that product contained too little active drug and too much placebo. About 50,000 faulty packets were distributed across Canada before the problem was discovered.
A law firm in Thunder Bay, Ont. launched an $800-million class action lawsuit against Alysena’s maker, Apotex, after more than 100 women came forward to say they had become pregnant while taking Alysena or endured undue stress.
With files from CTV Atlantic's Alyse Hand and CTVNews.ca Staff